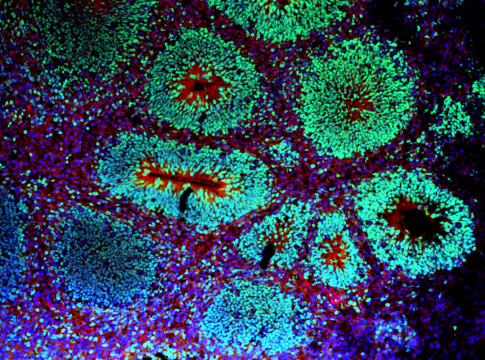
Abstract Astrocytes are not a uniform population but exhibit diverse morphological, molecular, and functional characteristics. However, how this diversity originates and becomes establishes during development, remains largely unknown. Here, using single-cell RNA sequencing and spatial transcriptomics, we identify five astrocyte subtypes with unique molecular features, spatial distributions and functions in the mouse neocortex and characterize essential regulators for their formation. Using TrackerSeq to trace clonally related astrocytes, we identify two distinct lineages that give rise to these five subtypes. One lineage derives from Emx1 + radial glial cells that initially generate neurons and later switch to astrocyte production. The other, with minimal neuronal output, predominantly produces a distinct subset of astrocytes marked by Olig2. Olig2 knockout disrupts lineage specification, leading to changes at molecular, morphological and functional levels. These findings shed light on the cellular mechanisms underlying astrocyte diversity, highlighting the presence of multiple radial glial cell subtypes responsible for generating cortical astrocyte subtypes.
Publication scientifique
Dual lineage origins contribute to neocortical astrocyte diversity
Autres publications de la plateforme
Developmental emergence of cortical neurogliaform cell diversity
Molecular programs guiding arealization of descending cortical pathways
Developmental emergence of first- and higher-order thalamic neuron molecular identities
Regional differences in progenitor metabolism shape brain growth during development
Single-cell genotyping and transcriptomic profiling of mosaic focal cortical dysplasia
Targeting pathological cells with senolytic drugs reduces seizures in neurodevelopmental mTOR-related...
Journal de publication
Auteurs:
Date de publication:
Plateforme:
Études récentes de la plateforme
Reprogrammer des cellules humaines pour étudier les défauts du cerveau

Suivi de la dopamine dans la maladie de Parkinson